



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Alkali Metal |
 Glossary Index |
 Alopecia |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
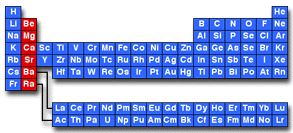
An alkaline earth is any element in the second column of the periodic table. These elements include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra):
Do not confuse this term with the word alkaline which refers to a basic substance (one with a pH greater than 7) or with alkali metal.
The second column of the periodic table is called Group 2 under IUPAC nomenclature and is is also called Group II or Group IIA using older naming conventions.
The alkaline earth elements are found in many common materials. For example, calcium ions are a major component of your bones, magnesium is used in a variety of metal alloys, strontium is used in television tubes, and barium compounds are used as X-ray contrast agents. Radium is a very rare radioactive material. Beryllium is a toxic and carcinogenic material that requires specialized handling.

Get your Class D and other specialty extinguishers from Safety Emporium.
Ca, Sr and Ba are reactive towards air and water in their elemental (metal) form, but much less so than their alkali metal counterparts. This reactivity increases as one moves down the column from Ca to Ba. The reaction of elemental Ca, Sr and Ba with water can be summarized by the chemical equation below where M = an alkaline earth metal.
M(s) + 2 H2O  M2+(aq) + 2 HO-(aq) + H2(g)
M2+(aq) + 2 HO-(aq) + H2(g)
In some cases, the heat of this reaction can ignite the hydrogen gas (H2) that is evolved in the reaction.
Although these metals are shiny when cut, they tend to tarnish in air. Calcium barium, and strontium should be stored under an inert atmosphere and/or mineral oil.
Note that there is a great difference between an alkaline earth metal (such as Ca) and an alkaline earth cation (such as Ca2+). Hazards often depend on the chemical state of the material!
The Safety Data Sheet for a material will contain vital information concerning the hazards of the material. These will depend on the specific alkaline earth and its form.
Beryllium is toxic in both the elemental form and in salts/compounds as Be2+, so special precautions such as respirators need to be used whenever dusts or fumes of Be or its compounds can be released. See the links below for further information. The other alkali metals are all flammable and water reactive, so special care needs to be taken in using these and a proper Class D extinguisher should be available.
Elemental Mg, Ca, Ba and Sr are incompatible with certain classes of chemicals such as halogenated solvents. See the notes under alkali metals for more info.
See also: alkali metal, halogen, inert, water reactive.