



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Sternutator |
 Glossary Index |
 Stupor |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Standard Temperature and Pressure (STP) is defined as 0 degrees Celsius and 1 atmosphere of pressure.
Do not confuse STP with the STP Products Company, a maker of oil and fuel additives...unless you are reading one of their Safety Data Sheets!
STP is used in many thermodynamic calculations and tabulations. Certain properties of matter such as density, viscosity, boiling point etc. will vary with changes in temperature or pressure. Having one common set of conditions ("state") for tabulating these values makes comparisons possible and eases calculations.
The concept of matter in its standard state (also called "standard conditions") is closely related. "Standard state" does not generally imply a specific temperature, but 25 °C (298 K) is most often used:
| State of Matter | Standard State |
|---|---|
| Gas | 1 atm of pressure |
| Liquid | Pure liquid |
| Solid | Pure solid |
| Solution | 1 molar |
| Elements | The most stable allotrope at STP, with  Gf0 = 0 Gf0 = 0 |
Many chemistry calculations are for materials that are in their standard state. One very useful rule for gases that does not necessarily require standard state conditions is the Ideal Gas Law (eq 1).
 | (1) |
Where:
| Symbol | Meaning | Typical Unit |
|---|---|---|
| P | Pressure | atm |
| V | Volume | liter |
| n | # of moles of material | mole |
| R | The Ideal Gas Constant | 0.08206 L.atm.mol-1.K-1 |
| T | Temperature | Kelvin |
Using the Ideal Gas Law, one can determine the value of any one of the four variables (P, V, n, T) if we know the value of the other three (R is a constant).
For example, 1,000 grams (1 kilogram = 2.2 pounds) of ethylene (which has a molar mass of 28 grams/mol) will occupy a volume of 800 liters at STP :
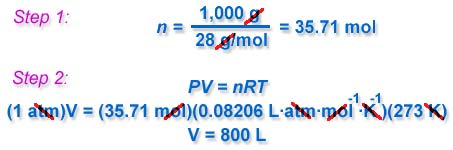
If we wished to calculate this volume at another, non-standard state temperature, such as 100 °C, we would simply substitute 373 K for the temperature (100 °C = 373 K) in the above calculation. The Ideal Gas Law is a very handy equation for estimating gas properties at both standard and non-standard conditions.
Safety Emporium has all kinds of lab equipment such as stirring hot plates.
The letters "STP" occur most commonly on a Safety Data Sheet after a physical property such as the density , flammable limit, or vapor pressure of the material. These properties are found in Section 9 (physical and chemical properties) of the SDS.
Remember: STP is 0 °C, NOT room temperature. Usually the properties at STP versus room temperature vary by less than 10% for gases and even less for liquids or solids. For example, in the calculation in the previous section, the volume would be 858 liters at 20 °C, a difference of 7%. And the density of water is 0.99987 g/ml at 0 °C versus 0.99823 g/ml at 20 °C, a difference of 0.16%.
However, sometimes the differences can be extreme - dimethylamine is a volatile liquid with a specific gravity of 0.680 at STP, but it is a gas above its boiling point of 7 °C!
See also: mass units, mole, pressure units
Additional definitions from Google and OneLook.
Entry last updated: Monday, January 16, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.