



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Hydrocarbon |
 Glossary Index |
 Hypergolic |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

We carry laboratory drying tubes and related equipment at Safety Emporium.
A hygroscopic material (literally "water seeking") is one that readily absorbs water (usually from the atmosphere).
In most cases, the water can be removed from the material by heating (sometimes under vacuum or under a flow of dry gas such as nitrogen).
Hygroscopic substances that are used to remove water from the surroundings are called desiccants. For example, those small packets marked DO NOT EAT that come with items such as electronic goods contain silica gel to absorb atmospheric moisture and prevent it from condensing on the product when the temperature falls.
In the laboratory, anhydrous hygroscopic substances such as calcium chloride (CaCl2), magnesium sulfate (MgSO4), and sodium sulfate (Na2SO4) are used to remove residual water from organic solutions. In this application, the solid is added to the solution. After a few minutes, the solid desiccant (now partially hydrated) is removed by filtration or decanting and then disposed of.

Get your water-reactive signs and labels at Safety Emporium.
Hygroscopic drying agents are also used in desiccators, sealed jars that have a layer of desiccant in the bottom. They are also used in drying tubes which are stuffed with the desiccant material and placed over the opening of a chemical apparatus to keep atmospheric moisture out. When the desiccant can no longer absorb water it is typically reactivated by heating and/or placing it under vacuum to regenerate the starting anhydrous form:
MgSO4(s) + 7 H2O 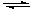 MgSO4•7H2O(s)
MgSO4•7H2O(s)
Others hygroscopic drying agents chemically react with water to form new substances and are not easily reversible. For example, sodium and potassium metal are used to scavenge residual water from organic solvents to reduce the water content to the ppm level. The reaction forms sodium or potassium hydroxide and hydrogen gas. This would be exceedingly dangerous if large amounts of water were present so the solvents are normally pre-treated to remove bulk water first:
2 Na(s) + 2 H2O → 2 NaOH(s) + H2(g)
Likewise phosphorous pentoxide, P2O5 (which actually occurs as P4O10), reacts with water to form phosphoric acid:
P4O10 + 6 H2O → 4 H3PO4
Hygroscopic materials are fairly common. Some may absorb a finite amount of water (such as magnesium sulfate, MgSO4) while others may attract so much water that they form a puddle and dissolve (deliquesce). For example, solid sodium hydroxide (NaOH) pellets will form a small corrosive puddle in less than an hour in moist air. The CU Boulder link below has a great picture of this phenomenon. Always be sure to clean up any spills of hygroscopic materials right away. Also be aware that hygroscopic materials typically release a large amount of heat when mixed with water.
Therefore, always ready the Safety Data Sheet to see what special storage procedures are required. Depending on the nature of the material one might store hygroscopic materials in a tightly sealed container, under vacuum, in a desiccator, or even in an inert atmosphere drybox.
Know their physical properties of the material (found in Section 9 of the SDS) so that if you open a container you can tell if the material has been contaminated with water (i.e. that jar of calcium chloride, CaCl2, should be a solid, not a liquid). Consult Section 7 (handling and storage) for recommendations on storage.
See also: Anhydride, anhydrous, water reactive.
Additional definitions from Google and OneLook.
Entry last updated: Monday, January 16, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.