



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Mist |
 Glossary Index |
 Mole |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
Chill out with laboratory condensers from Safety Emporium.
According to the 2012 version of the OSHA Hazard Communication Standard, 29 CFR 1910.1200 (HCS 2012), a mixture "means a combination or a solution composed of two or more substances in which they do not react".
A chemist defines a mixture as a combination of two or more substances in which each substance retains its own chemical identity and properties.
In general, mixtures have no fixed composition. The amount of one or more components (substances) can usually vary over a wide range.
Mixtures generally fall into one of two categories:
An important property of mixtures is that they can (usually) be separated into their individual components without requiring any chemical reactions. For example, we can evaporate our sugar-water solution to obtain pure water or pure sugar. Likewise, we could put our chocolate chip cookie into water to dissolve away the dough and collect pure chocolate chips.
When chemicals are combined, they can either form a mixture or they can chemically react to form new chemical species. For example, consider atoms A (blue) and B (red). If they do not react chemically, we obtain a mixture:
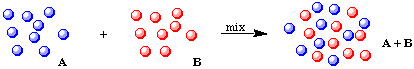
If each atom of A reacts with exactly one atom of B, we can form a new molecule, AB, that contains one atom of A chemically bonded to one atom of B. This is a chemical reaction because the new material will have different physical and chemical properties than either A or B.
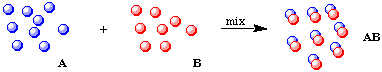
Sometimes, we might form AB but have some A and/or B left over (the reaction might be reversible, or we may have put in more of one component). In these cases, one or more of the starting materials (A and/or B) as well as the new chemical species (AB) will be present in our resulting mixture. This is sometimes called an incomplete reaction:
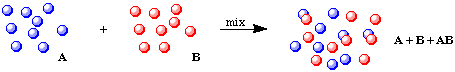

Get your GHS-compliant labels and signs from Safety Emporium.
HCS 2012, introduced the concept of hazard classification in which both the health and physical hazards of the material must be assigned using a rigorous process outlined in Appendix A for health hazards and in Appendix B for physical hazards. Appendix A lists general considerations for the classification of mixtures in paragraph A.0.4.1:
(a) Where test data are available for the complete mixture, the classification of the mixture will always be based on those data;
Where test data are not available for the mixture itself, the bridging principles designated in each health hazard chapter of this appendix shall be considered for classification of the mixture;
If test data are not available for the mixture itself, and the available information is not sufficient to allow application of the above-mentioned bridging principles, then the method(s) described in each chapter for estimating the hazards based on the information known will be applied to classify the mixture (e.g., application of cut-off values/concentration limits).
Manufacturers are required to perform this hazard classification and as well as issue a Safety Data Sheet and GHS-compliant label. The process can be quite involved for mixtures so professional assistance is suggested if this is beyond the manufacturer's expertise.
Mix things up with lab equipment such as stirring hot plates from Safety Emporium.
See also: chemical formula, mole and molecular weight.
Additional definitions from Google and OneLook.
Entry last updated: Tuesday, January 3, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.