| The Home page of ILPI's Safety Data Sheet (SDS) Resource, the leader in SDS information since 1995! | |
| The history and philosophy behind this resource. | |
| A curated collection of books and reference materials concerning Safety Data Sheets and closely related topics. | |
| Paste your plain text SDS into the SDS-Demystifier, and it will be converted into a hypertext-enriched document with links to detailed explanations of each key term. | |
| An extensive list of frequently asked questions about Safety Data Sheets including regulations, content, compliance, and more. | |
| A humorous take on Safety Data Sheet jargon. Fill in the blanks on our entry form to generate a personalized Unsafety Data Sheet to share with your coworkers. | |
| Since 1995, we've maintained this massive curated list of the best places to find Safety Data Sheets on the Internet. | |
| You are here! Way more than a glossary, this hypertext-enhanced resource covers hundreds of SDS-related terms and expert knowledge. Each entry includes both the SDS relevance and links to additional authoritative resources. | |
| Archived results of Safety Data Sheet related polls taken by some of our millions of site visitors | |
| The OSHA regulations behind SDS regulations, including the inspection guidelines and over 400 official interpretations letters under the Hazard Communication Standard | |
| Commercial suppliers of SDS authoring and management software as well as cloud compliance services. | |
| Commercial companies that will create SDS's for your specific needs as well as SDS translation companies. |

Safety signs, banners, and scoreboards? Get yours at Safety Emporium!
Definition
- Boiling point is the temperature at which a liquid changes to a gas (vapor) at normal atmospheric pressure.
A more specific definition of boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. - The normal boiling point is the temperature at which the liquid boils when the external pressure is one atmosphere (760 torr = 760 mm Hg = 1 atm = 101.3 kPa = 14.7 psi).
- Pure substances have a discrete boiling point. Mixtures consisting of two or more chemicals can have a single boiling point, but depending how the components interact, boiling may instead occur over a range of temperatures and the term boiling point range is a better descriptor. For example, petroleum ether (a mixture of various aliphatic hydrocarbons) typically has a boiling point range of 42 to 62 °C (108 to 144 °F) .
Additional Info
How can one determine the temperature at which water boils? After all, bubbles start to appear in water well below the known boiling point of 100 degrees C (212 °F).
The answer lies in monitoring the temperature of the material with time. When the boiling point is reached, the temperature will not rise again until all of the liquid has evaporated. This is due to the high heat capacity of water (it takes much more energy to convert water from liquid to gas than it does to raise the temperature of liquid water).
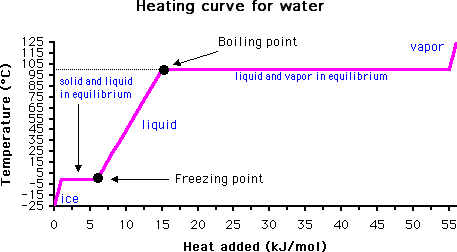
Of course, if water is heated under pressure this may raise the boiling point above its normal boiling point of 100 degrees C. Likewise, the addition of a solute may also raise the boiling point, a phenomenon called boiling point elevation (see Further Reading below for more information).
Not all substances have a boiling point. Some substances may decompose into other materials when heated instead of boiling. Wood does not boil, and neither does calcium carbonate, which decomposes to calcium oxide and carbon dioxide when heated. Other substances, such as solid carbon dioxide, may sublime to give gases without ever forming a liquid under normal conditions. However, under higher pressure, carbon dioxide will turn to liquid and then boil as the temperature is raised. Therefore, it is a best scientific practice to always report the external pressure when reporting a boiling point.
SDS Relevance
If applicable and known, the boiling point of a material is required to appear in Section 9 (physical and chemical properties) of the material's Safety Data Sheet.
Knowing the boiling point of a substance is an important consideration for storage. For example, storing a chemical with a boiling point of 50 oC (122 oF) in direct sunlight or next to a boiler could cause the material to completely vaporize and/or result in a fire or explosion.
Items with a low boiling point generally have a high vapor pressure. Containers of such material can build up signicant pressure even when they are below their boiling point. Likewise, low-boiling materials easily produce large amounts of vapor which can be flammable or even explosive.

Reactions run more easily with laboratory heating mantles and temperature controllers from Safety Emporium.
Further Reading
- Melting Point, Freezing Point, Boiling Point at Purdue University.
- Boiling point and related concepts at Carl Nave's HyperPhysics site.
- Wikipedia's entry for boiling point.
- ChemSpider has information on over 63 million structures and includes all kinds of data such as boiling points (search and then click on the Properties tab in the result).
- The International Boiling Point Project - a great K-12 experiment. Available thanks to the Internet Archive.
- Safety Emporium sells a variety of Thermometers and Thermocouples for accurate temperature measurement in the laboratory.
See also: evaporation rate, flash point, freezing point, vapor pressure.
