
While beta-hydride and alpha-elimination reactions are the most common elimination reactions in transition metal chemistry, other elimination modes are also possible. Eliminations from the gamma, delta or epsilon position of a coordinated ligand are also observed, most commonly with late transition metal complexes but also in complexes that are sterically and electronically unsaturated. A few examples of each of these are shown below.
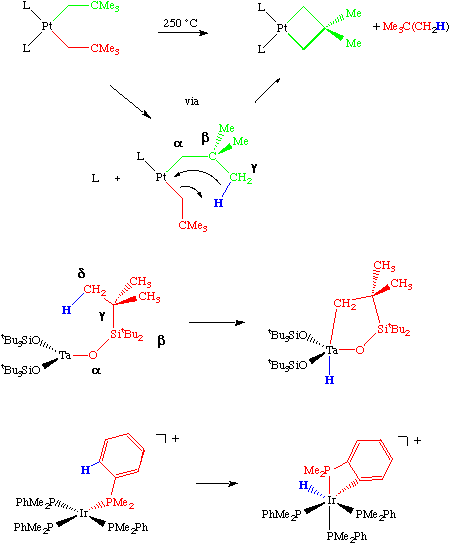
As shown in the above examples, this mode of reactivity is an important method for the synthesis of late transition metal metallacycles and is therefore sometimes referred to as cyclometallation. The last example shown is called a orthometallation reaction as it involves the activation of a C-H bond in the ortho position of an aryl group. This turns out to be an important degradation pathway in industrially desirable catalysts that contain phosphine ligands.
The mechanism of these reactions is essentially no different than that of an alpha-elimination which, in turn, is really just a variant of the oxidative addition reaction.