| The Home page of ILPI's Safety Data Sheet (SDS) Resource, the leader in SDS information since 1995! | |
| The history and philosophy behind this resource. | |
| A curated collection of books and reference materials concerning Safety Data Sheets and closely related topics. | |
| Paste your plain text SDS into the SDS-Demystifier, and it will be converted into a hypertext-enriched document with links to detailed explanations of each key term. | |
| An extensive list of frequently asked questions about Safety Data Sheets including regulations, content, compliance, and more. | |
| A humorous take on Safety Data Sheet jargon. Fill in the blanks on our entry form to generate a personalized Unsafety Data Sheet to share with your coworkers. | |
| Since 1995, we've maintained this massive curated list of the best places to find Safety Data Sheets on the Internet. | |
| You are here! Way more than a glossary, this hypertext-enhanced resource covers hundreds of SDS-related terms and expert knowledge. Each entry includes both the SDS relevance and links to additional authoritative resources. | |
| Archived results of Safety Data Sheet related polls taken by some of our millions of site visitors | |
| The OSHA regulations behind SDS regulations, including the inspection guidelines and over 400 official interpretations letters under the Hazard Communication Standard | |
| Commercial suppliers of SDS authoring and management software as well as cloud compliance services. | |
| Commercial companies that will create SDS's for your specific needs as well as SDS translation companies. |

Safety signs, banners, and scoreboards? Get yours at Safety Emporium!
Definition
The pH of a solution is the negative logarithm of the hydrogen ion (H+) concentration (in moles per liter = molarity). Chemists write "hydrogen ion concentration" as [H+].
pH tells you whether a solution is acidic, basic or neutral. The corresponding ranges are:
- Acidic - the pH is between zero and 7.0
- Neutral - the pH is 7.0
- Basic (also called alkaline) - the pH is between 7.0 and 14.
For strong acids or bases, these values may actually be higher than 14 or lower than 0, but the 0-14 range is most commonly encountered.
Additional Info
The pH scale is logarithmic. That means each change of one in pH value is 10 times more or less acidic. Therefore, a substance with a pH of 2 is 1,000 times more acidic than one with a pH of 5.
The pH values of some common substances are given in the table below.
| Substance | Typical pH | [H+], M |
|---|---|---|
| Stomach acid (gastric juices) | 1.4 | 0.0398 |
| Lemon juice | 2.4 | 0.00398 |
| Vinegar | 3.0 | 1 x 10-3 |
| Tomatoes | 4.2 | 6.31 x 10-5 |
| Water exposed to air | 5.5 | 3.16 x 10-6 |
| Pure water | 7.0 | 1 x 10-7 |
| Blood or tears | 7.4 | 3.98 x 10-8 |
| Baking soda | 8.4 | 3.98 x 10-9 |
| Household ammonia | 11.5 | 3.16 x 10-12 |
| Household bleach | 12.5 | 6.31 x 10-13 |
pH can be measured in many different ways. A simple example is litmus paper which can tell you if a solution is basic or acidic. Other fast and inexpensive indicator papers and reagents can also be used. Examples include phenolphthalein, bromophenol blue, methyl red and gentian violet (see Further Reading below for more). Electronic pH meters can provide very accurate pH determinations over a wide range of hydrogen ion concentrations, but cost more than simple indicators.
Finally, note that the concept of pH is technically/generally correct only for dilute solutions (say, 0.001 M and below). As the concentration of a dissolved substance, in this case, H+ becomes greater, chemists use a concept called activity instead. This is because ions have charge, and when there are enough ions in the solution, they begin to interact in ways that may affect their availability to interact with other species. But for the purposes that most of need on an MSDS, pH works just fine.
SDS Relevance
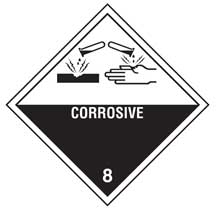
Safety Data Sheets will list the pH of a substance, if known and applicable, in Section 9 (physical and chemical properties). It is important to know the pH of substances that may be corrosive or could react with incompatible materials; such information will be found in Section 10 (stability and reactivity). For example, acids and bases should not be stored or used near each other as their accidental combination could generate a huge amount of heat and energy, possibly resulting in an explosion. Section 6 (accidental release measures) will provide critical information if the material is spilled.
pH is also important to know in case you spill the material on your skin or eyes. Whenever a substance enters the eye, flush with water for 15 minutes and get prompt medical attention. If a basic substance enters the eye this is particularly important as basic materials tend to cause worse eye damage and are harder to flush out of the eye tissues than acidic materials.
Further Reading
- Acids and Bases at Wyzant (formerly ChemTutor.com).
- Acid-Base Chemistry at Shodor.org.
- Acids, Bases and Salt - Background and Naming at Teachers.Net.
- How sensors work - pH measurement at sensorland.com.
- pH Measurement FAQs at Cole-Parmer, a supplier of pH measurement tools.
- The Molecular Basis of Indicator Color Changes at General Chemistry Online! Also see Acids and bases: Frequently asked questions which includes indicators.
- Acid-Base Indicators at Chemguide.
- Chembuddy discusses activity vs. pH.
- Standard Operating Procedure for Corrosive Chemicals at the University of Pennsylvania.
- Spill Response Guide: Corrosive Acids at the University of Iowa; the current version is behind a firewall.
- Guide for Chemical Spill Response at the American Chemical Society (ACS).